In the medical diagnostics space, understanding the severity of a concussion has always been a grey area resulting in expensive CT scans that are necessary in only about 8 percent of the cases. Despite this known issue, the number of avenues to detect the seriousness of traumatic brain injuries (TBIs) is limited, at least until now. Enter BioDirection, a privately held company that is on a mission to deliver a precise TBI diagnostics solution through the detection of biomarkers found in the bloodstream after injury. BioDirection has designed nanosensors using nanowires that are 1/10,000 the size of a human hair.
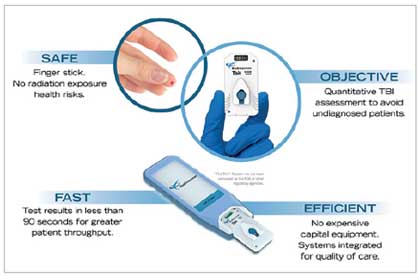
BioDirection offers a ground-breaking solution designed to detect concussion and traumatic brain injury quickly and accurately. The company’s Tbit™ System delivers precise results in real-time, enabling medical professionals to determine the appropriate treatment for the patient, without the utilization of CT scan. Sharad Joshi, President and CEO of BioDirection, adds, “Our cutting-edge technology provides a test result in 90 seconds from a finger stick of blood, and is accessible to a huge market that spans across sports, military training and deployment, senior living facilities, and hazardous work environments.” Additionally, this technology delivers objective information to aid in the diagnosis of the concussion, reducing unnecessary exposure to the radiation of CT scans.
The Tbit™ System is based on a proprietary silicon nanosensor that detects and measures protein biomarkers released following a brain injury. The platform includes a small portable analyzer and a single-use disposable cartridge that can measure biomarkers from a single drop of blood. Collectively, the Tbit™ System addresses three vital pre-requisites needed for streamlined TBI detection: ease-of-use, portability, and cost-effectiveness. The nanosensor built into the devices has several unique features including direct, label-free and real-time measurement of biomarkers, setting it apart from other diagnostic tools in the market. This primary focus on the point of care is the overarching philosophy behind the development of the Tbit™ System, which is portable, battery-operated, and seamlessly connects to the internet while adhering to HIPAA compliance guidelines.
Such an approach to TBI detection brings in the much-needed versatility for the device, accelerating the scientific understanding of the role that specific biomarkers play in TBI. Device connectivity enables physicians and healthcare professionals to obtain more information about new detection algorithms in real-time and accelerates scientific understanding of diseases. Furthermore, advancements in biomarkers would open-up new opportunities in the diagnosis of a wide array of ailments within the medical sciences. For instance, the Tbit™ System could be a potential game-changer for the diagnosis of stroke, Alzheimer’s disease, and similar brain-related ailments. In the foreseeable future, the Tbit™ System may prove instrumental in supporting a full continuum of care ranging from stratification of injury, prognosis, and return to play stages. As for today, BioDirection is laying the groundwork for the future. The company has decided to establish in-house manufacturing of the sensor technology, in order to ensure that its products meet all necessary criteria for consistency and reliability. CEO Sharad Joshi shared, “We anticipate that our new facility will be fully operational within the next several months.” This plant will be a critical step forward in the availability and commercialization of the Tbit™ System. BioDirection anticipates entering the European and the U.S. markets in the year 2020, following CE mark and FDA clearance.
